Abstract
Background: Treatment with chimeric antigen receptor T-cell (CAR-T) therapies has been associated with durable clinical responses in patients with relapsed/refractory B-cell malignancies. However, these treatments can be complicated by cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Janus kinase (JAK) pathways are important for the cytokine signaling involved in CRS and ICANS pathogenesis. Itacitinib, a potent, selective, oral JAK1 inhibitor, was evaluated to manage onset and severity of CRS and ICANS in response to CAR-T infusion in a phase 2 clinical trial (NCT04071366). Preliminary results demonstrated that prophylactic itacitinib treatment in patients receiving CAR-T therapy resulted in reduced onset and severity of CRS and ICANS (Frigault M et al, abstr #OS15-07, European Society for Blood and Marrow Transplantation Annual Meeting [EBMT] 2022). To assess whether JAK inhibition affects CAR-T expansion and function, circulating CAR-Ts were analyzed from patient samples from the phase 2 trial.
Methods: Whole-blood peripheral blood mononuclear cells were collected before and during itacitinib treatment (200 mg once daily) and from the peri-CAR-T infusion period. CAR-T levels were quantitated using a droplet digital polymerase chain reaction (PCR) assay (Bio Rad, Hercules, CA). CAR-Ts were visualized using RNAScope technology (Advanced Cell Diagnostics [ACD], Newark, CA). Functional readouts were based on interferon (IFN)-g and granzyme B expression and were quantitated by HALO® image analysis platform (ACD). Serum cytokine analysis was performed using a fully automated microfluidic immunoassay system (Protein Simple Ella, San Jose, CA).
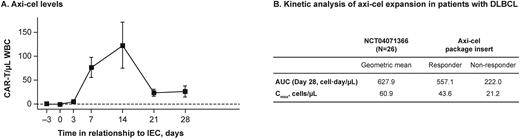
Results: Thirty-one patients received itacitinib before infusion with axicabtagene ciloleucel (axi-cel); of these, 28 were diagnosed with diffuse large B-cell lymphoma (DLBCL). Expansion of CAR-Ts was quantitated and normalized to total white blood cell (WBC) counts and plotted over time (Figure 1A). Peak levels of CAR-Ts were observed between 7 and 14 days postinfusion in the presence of a JAK inhibitor, consistent with published CAR-T expansion data. Kinetic analysis of patients with DLBCL (Figure 1B) resulted in area under the curve and maximum observed concentration levels comparable to historical values. RNAScope technology allowed visualization of CAR-Ts in patient samples at Day 7 or 14 postinfusion, and the cells expressed functional markers IFN-g and granzyme B. Systemic inflammatory cytokine levels, including interleukin (IL)6, IFN-g, IL2, and granulocyte-macrophage colony-stimulating factor, were increased as early as 1 to 3 days after CAR-T infusion and correlated with CRS and ICANS grades.
Conclusion: Patients receiving commercial CAR-T products co-treated with itacitinib demonstrated kinetic parameters of CAR-T expansion similar to historical values. Functional marker expression and cytokine production suggest that JAK inhibition at the current study dose has no deleterious effect on CAR-T function. The current study will expand with a randomized, double-blind, placebo-controlled portion that will enroll patients with DLBCL receiving axi-cel with itacitinib (200 mg twice daily) vs placebo. The assays described will be used to further investigate CAR-T proliferation, persistence, and function in the presence of either itacitinib or placebo.
Disclosures
Pratta:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Burke:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. DiPersio:BioLineRx, Ltd.: Research Funding; Macrogenics: Research Funding; NeoImmune Tech: Research Funding; Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Maziarz:ASTCT: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Consultancy, Honoraria; Novartis: Other: Support for research on CART; Allovir: Other: Support for research on Allo HCT costs of care of infectious related complications; Orca Bio: Other: Support for research analysis and for medical writing. Feldman:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Brodeur:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Timmers:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Ivanova:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Barbour:Incyte Corporation: Ended employment in the past 24 months; Karyopharm: Current Employment, Current equity holder in publicly-traded company. Morariu-Zamfir:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Frigault:Cytoagents: Consultancy; Iovance: Consultancy; Novartis: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding; Arcellx: Research Funding; JnJ/Legend: Consultancy; BMS: Consultancy.
OffLabel Disclosure:
Itacitinib, a potent, selective, oral JAK1 inhibitor, was evaluated to manage onset and severity of CRS and ICANS in response to CAR-T infusion in a phase 2 clinical trial
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal